
Research by Otto Hahn, Lise Meitner, Enrico Fermi and others, such as J. Eugène-Melchior Péligot was the first person to isolate the metal and its radioactive properties were discovered in 1896 by Henri Becquerel. The 1789 discovery of uranium in the mineral pitchblende is credited to Martin Heinrich Klaproth, who named the new element after the recently discovered planet Uranus. It was also used for tinting and shading in early photography. Uranium glass fluoresces green in ultraviolet light. Uranium is used as a colorant in uranium glass, producing lemon yellow to green colors. Depleted uranium ( 238U) is used in kinetic energy penetrators and armor plating.

This generates the heat in nuclear power reactors, and produces the fissile material for nuclear weapons. In sufficient concentration, these isotopes maintain a sustained nuclear chain reaction. Uranium-238 has a small probability for spontaneous fission or even induced fission with fast neutrons uranium-235 and to a lesser degree uranium-233 have a much higher fission cross-section for slow neutrons. Another fissile isotope, uranium-233, can be produced from natural thorium and is studied for future industrial use in nuclear technology. Uranium-238 is fissionable by fast neutrons, and is fertile, meaning it can be transmuted to fissile plutonium-239 in a nuclear reactor. However, because of the tiny amounts found in nature, uranium needs to undergo enrichment so that enough uranium-235 is present. Uranium-235 is the only naturally occurring fissile isotope, which makes it widely used in nuclear power plants and nuclear weapons. Many contemporary uses of uranium exploit its unique nuclear properties. The half-life of uranium-238 is about 4.47 billion years and that of uranium-235 is 704 million years, making them useful in dating the age of the Earth. Uranium decays slowly by emitting an alpha particle. It occurs naturally in low concentrations of a few parts per million in soil, rock and water, and is commercially extracted from uranium-bearing minerals such as uraninite. Its density is about 70% higher than that of lead, and slightly lower than that of gold or tungsten. Uranium has the highest atomic weight of the primordially occurring elements. The most common isotopes in natural uranium are uranium-238 (which has 146 neutrons and accounts for over 99% of uranium on Earth) and uranium-235 (which has 143 neutrons).
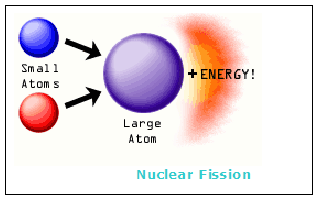
Uranium is weakly radioactive because all isotopes of uranium are unstable the half-lives of its naturally occurring isotopes range between 159,200 years and 4.5 billion years. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons.
Nu laser fission uranium series#
It is a silvery-grey metal in the actinide series of the periodic table. Uranium is a chemical element with the symbol U and atomic number 92.


 0 kommentar(er)
0 kommentar(er)
